Abstract
Neurofibromatosis type 1 (NF-1) is an autosomal dominant disorder affecting approximately 1:3000 individuals globally. While approximately 50% are familial, with over 3000 causative germline variants in the neurofibromatosis (NF1) gene identified, the remainder occur sporadically. These mutations lead to haploinsufficiency of NF1 and neurofibromin, a tumor suppressor and important negative regulator of RAS signaling. Children with NF-1 have a higher risk of developing juvenile myelomonocytic leukemia and acute myeloid leukemia, but rarely develop acute lymphoblastic leukemia (ALL).
A 9-year-old male presented in 2015 with persistent migratory subcutaneous swellings and multiple bony aches with lytic lesions on bone imaging. He had a high white cell count with eosinophilia (WCC 43.4 x 10 9/L, eosinophils 23.87 x 10 9/L) with no circulating blasts, 10% marrow blasts (CD10+/CD19+/CD34+) and was CNS negative. Although previously undiagnosed, NF-1 was clinically suspected due to typical skin changes.
He was diagnosed with iAMP21 ALL and NF-1 was confirmed with the identification of a germline NF1 donor splice site mutation (c.1845G>A:p.L615=). Bone marrow cells were sorted by flow cytometry on CD19 positivity and underwent transcriptomic sequencing. This revealed a P2RY8-CRLF2 gene fusion, with no other clinically relevant variants, while a custom Taqman low density array indicated high-risk B-ALL subtype Ph-like ALL. Multiplex ligation-dependent probe amplification (MLPA) confirmed iAMP21 and also identified IKZF1 exon 2-3 and BTG1 deletions.
Treatment followed the high-risk B-ALL arm of the AEIOP-BFM ALL2009 protocol due to persistent end-consolidation MRD in addition to iAMP21 and the Ph-like phenotype. He relapsed three years later off treatment and was refractory to both salvage chemotherapy and blinatumomab. The iAMP21, P2RY8-CRLF2 gene fusion, IKZF1 exon 2-3 and BTG1 deletions remained detectable. Whole exome sequencing of CD19 positive samples from diagnosis, relapse and mesenchymal stem cells (germline control) was performed, identifying a NF1 c.7400dupT:p.L2467 frameshift (fs) mutation only at relapse.
To understand the implications of NF1 p.L2467fs, the P2RY8-CRLF2 gene fusion was first transduced into the interleukin 3 (IL3) dependent murine pro-B cell line Ba/F3. P2RY8-CRLF2 alone is not transforming and is thought to be a secondary event in iAMP21 ALL, providing an ideal model to study the cumulative effect of the NF1fs. The NF1fs was then introduced to the P2RY8-CRLF2 cells by CRISPR/Cas9. A proliferation assay was performed without IL3 and demonstrated the P2RY8-CRLF2+NF1fs cell line was IL3 independent, indicative of leukemic transformation, whereas all other lines were not (vs Ba/F3, p = 0.001).
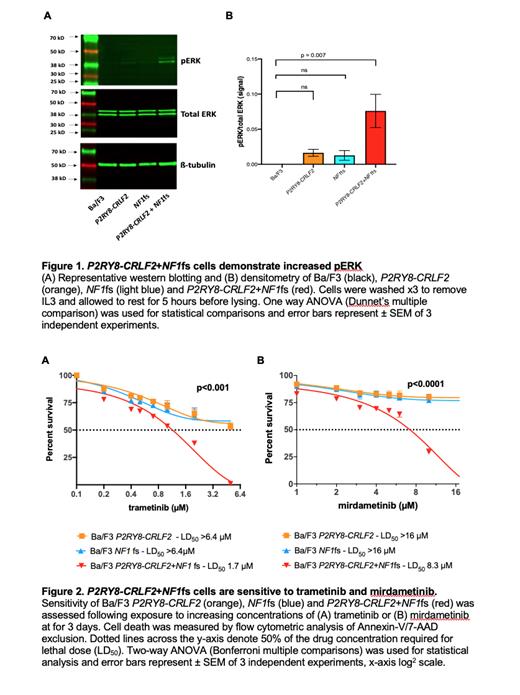
Neurofibromin can be constitutively phosphorylated at the c-terminus, negatively regulating NF1-GAP activity, suppressing RAS signaling and inducing cell cycle arrest. Therefore, to demonstrate loss of function due to the c-terminus NF1 p.L2467fs and increased RAS signaling, western blotting for pERK was performed. Significant upregulation of pERK was confirmed in P2RY8-CRLF2+NF1fs in comparison to Ba/F3 control cells (p=0.007) (Figure 1).
The MEK inhibitors trametinib and mirdametinib are in clinical trials for NF-1 patients and have shown efficacy in ALL models with RAS mutations. In a 3-day cell death assay, only P2RY8-CRLF2+NF1fs demonstrated sensitivity to trametinib (LD 50 P2RY8-CRLF2 = >6.4 µM, NF1fs = >6.4 µM, P2RY8-CRLF2+NF1fs =1.7µM; p < 0.001) and mirdametinib (LD 50 P2RY8-CRLF2 = >16 µM, NF1fs = >16 µM, P2RY8-CRLF2+NF1fs = 8.3 µM; p < 0.0001) (Figure 2).
Here, we have demonstrated a LOF NF1fs mutation using an in-vitro model of ALL. Germline NF1 haploinsufficiency and a second hit NF1 mutation in ALL is limited to one report of monozygotic twins with neurofibromatosis. We propose that NF1 p.L2467fs caused bi-allelic LOF and therefore contributed to relapse in this patient. An understanding of the genomic complexities that lead to relapse may also inform personalized treatment strategies. While this patient subsequently achieved remission with inotuzomab and underwent successful stem cell transplantation, the sensitivity to MEK inhibitors is an exciting development for neurofibromatosis patients with ALL.
Yeung: Amgen: Honoraria; BMS: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Honoraria, Research Funding. White: BMS: Honoraria, Research Funding; Novartis: Research Funding.